ETAP Standard Methods
Relatively few of the tens of thousands of chemicals in commerce, as well as those found in the environment, various waste streams, and the human body have traditional toxicity data or epidemiological data that can inform assessments of risks to human health. The EPA Transcriptomic Assessment Product is intended to be applied to substances with no existing or publicly accessible repeated dose toxicity studies or suitable human evidence. The underlying methods and data analysis procedures of the ETAP are highly standardized and structured, and the decision context is narrowly focused on substances lacking data.
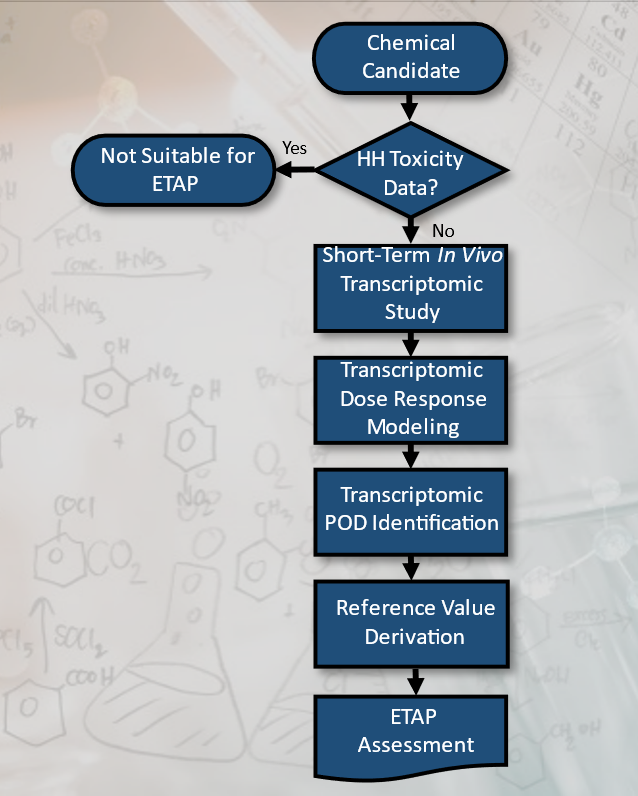
The ETAP consists of three primary components with associated processes and decision points within each component:
- Initial database searches and systematic evidence map development to ensure a chemical lacks suitable toxicity or human epidemiological studies;
- Short-term in vivo study and transcriptomic measurements to identify a point-of departure; and,
- Derivation of a reference value and reporting.
The results are reported in a standardized template and reviewed for quality prior to release.
For more details, read the report on the Standard Methods for Development of EPA Transcriptomic Assessment Products (ETAPs).
Additional Resources
- Overview and Existing Human Health Assessments (Society of Toxicology Annual Meeting 2024)
- New Additions to Address Important Gaps (Society of Toxicology Annual Meeting 2024)
- Scientific Studies Supporting Development of Transcriptomic Points of Departure for EPA Transcriptomic Assessment Products (ETAPs)
