Prioritization of Existing Chemicals Under TSCA
Prioritization is the initial step in the process of evaluating existing chemicals under the Toxic Substances Control Act (TSCA) and is codified in a final Chemical Prioritization Process rule. The purpose of prioritization is to designate a chemical substance as either high priority for immediate further risk evaluation, or low priority, for which risk evaluation is not warranted at the time.
TSCA further requires that upon completion of a risk evaluation (other than those requested by a manufacturer), EPA must designate at least one additional High-Priority Substance to take its place, thus ensuring that the EPA’s risk evaluation queue always remains full. Prioritization is a priority-setting step. High-Priority Substance designations are not indications of risk and Low-Priority designations are not indications of safety.
The following graphic provides an overview of EPA’s chemical prioritization process.
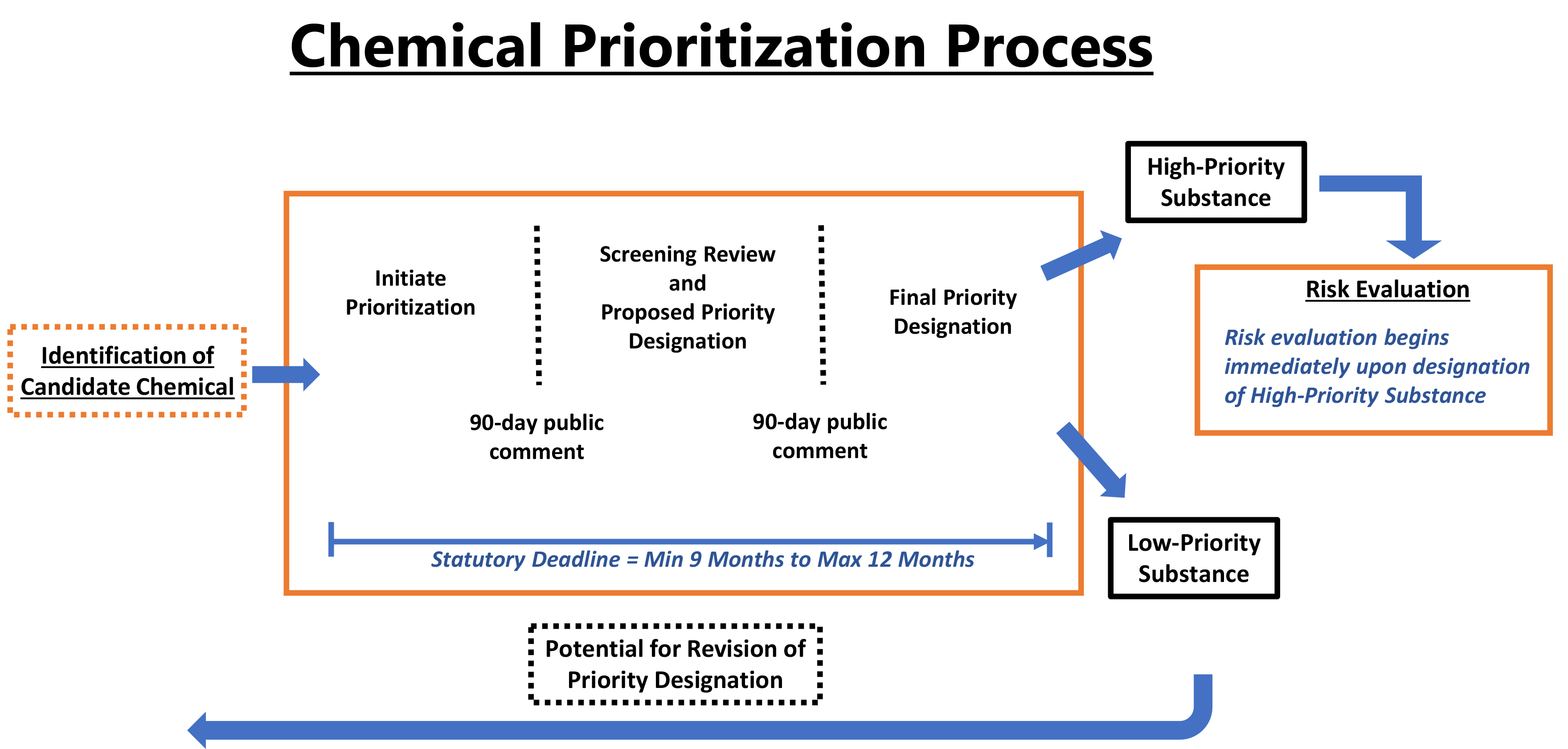
Learn more about the stages of EPA’s chemical prioritization process:
- Approach to prioritization
- Candidate selection
- Initiation
- Screening review
- Proposed designation
- Final designation
- Revision of designation
Approach to Prioritization
The general approaches EPA may consider for identifying existing chemicals as potential candidates for prioritization can be found in the Agency’s "A Working Approach for Identifying Potential Candidate Chemicals for Prioritization." The document lays out EPA’s near-term approach for identifying potential chemicals for prioritization, the initial step in evaluating the potential risk to environmental and human health of existing chemicals under TSCA. This document also includes a longer-term risk-based approach for managing the larger TSCA chemical landscape on the TSCA Inventory.
EPA has opened a chemical-specific public docket, one for each of the remaining chemicals on the 2014 Update to the TSCA Work Plan. See types of information EPA is seeking and learn how to submit the information to the dockets.
Candidate Selection
In identifying candidates for the prioritization process, TSCA requires that at least 50% of all chemical substances on which risk evaluations are being conducted by the Administrator are drawn from the 2014 Update of the TSCA Work Plan for Chemical Assessments and that EPA also give preference to Work Plan chemicals with the following characteristics:
- Persistence and bioaccumulation scores of three; and
- Known human carcinogens; and high acute chronic toxicity.
Aside from these statutory preferences and requirements, EPA has discretion to determine which chemical substances to prioritize.
Initiation
At the initiation step, EPA will formally announce a chemical substance the Agency plans to put through the prioritization process in a Federal Register Notice and give the public a 90-day comment period to submit potentially relevant information. Initiation formally begins the prioritization process and starts a nine to 12-month statutory time frame during which the Agency must designate the chemical substance as either a High- or Low-Priority Substance.
Screening Review
To support a proposed priority designation, EPA will screen the chemical substance under its conditions of use against certain criteria specified in TSCA section 6(b)(1)(A) by reviewing the reasonably available information with respect to:
- The hazard and exposure potential of the chemical substance;
- Persistence and bioaccumulation;
- Potentially exposed or susceptible subpopulations;
- Storage near significant sources of drinking water;
- The conditions of use or significant changes in the conditions of use of the chemical substance;
- The volume or significant changes in the volume of the chemical substance manufactured or processed; and
- Other risk-based criteria that EPA determines to be relevant to the designation of the chemical substance’s priority.
“Conditions of use” is a term in TSCA that means “the circumstances, as determined by the Administrator, under which a chemical substance is intended, known, or reasonably foreseen to be manufactured, processed, distributed in commerce, used or disposed of.” For purposes of prioritization, the Administrator may determine that certain activities fall outside the definition of “conditions of use.”
Additionally, during the Risk Evaluation scoping process, EPA will undergo a second process to identify the “conditions of use” that the Agency expects to consider during the evaluation. EPA may not exclude conditions of use from the scope of the risk evaluation. However, the conditions of use considered during the risk evaluation process may be the same or may differ from those identified during the prioritization process based on the information reasonably available at each stage. Learn more about the conditions of use in the risk evaluation processes.
Proposed Designation
At the proposed designation step, EPA will:
- Propose to designate a chemical substance as either a High-Priority Substance or a Low-Priority Substance;
- Publish the proposed designation and the information, analysis, and basis used to make the designation; and
- Take public comment on the proposed designation and supporting materials for 90 days.
The standards for designating chemicals as High- or Low-Priority Substances are as follows:
- High-Priority Substance – “a chemical substance that the Administrator concludes, without consideration of costs or other nonrisk factors, may present an unreasonable risk of injury to health or the environment because of a potential hazard and a potential route of exposure under the conditions of use, including an unreasonable risk to potentially exposed or susceptible subpopulations identified as relevant by the Administrator”
- Low-Priority Substance – “if the Administrator concludes, based on information sufficient to establish, without consideration of costs or other nonrisk factors, that such substance does not meet the [High-Priority] standard”
Final Designation
After considering public comments received on a chemical’s proposed designation, the final designation step requires EPA to:
- Finalize a High-Priority Substance designation and immediately initiate a risk evaluation; or
- Finalize a Low-Priority Substance designation determining that risk evaluation is not warranted at the time.
EPA’s final priority designation will be published in the Federal Register, along with the information analysis and basis used to support the designation.
Chemicals designated as High-Priority Substances through the prioritization process will enter the TSCA risk evaluation process. Find the docket numbers, problem formulations, scope documents and supplemental documents for each chemical substance undergoing risk evaluation under TSCA.
Revision of Designation
EPA may revise the designation of a Low-Priority Substance to a High-Priority Substance based on information available to the Administrator. The process for revising such a designation involves restarting the prioritization process, including re-initiating, re-proposing and re-finalizing a designation, and would necessarily provide the same opportunities for public comment.
