The Chalkboard Archive
Check out these highlights of EPA Children's Environmental Health Research previously posted on "The Chalkboard" webpage.
2025
July/August
Studying the Potential Impacts of Air Pollution on Young Hearts and Lungs
Anyone who has had the privilege to be in the delivery room when a child is born knows how carefully doctors and nurses watch for those first few breaths from a newborn. A couple of big gulps of air, followed by a robust outburst of crying are good indicators. Combined with a rich skin hue and it’s a good bet a healthy baby has arrived. Strong lungs and heart are key.
And because lung and heart development span from the earliest stages of life to well past those first few breaths, researchers are working to identify if exposure to air pollution during certain time periods along the way could lead to long-term health impacts. Pinpointing such “windows of susceptibility” could provide key information for protecting children’s environmental health.
Three recent EPA studies focused on providing just that kind of information.
Wildfire Smoke and Pediatric Prescriptions of Respiratory Medicine
With wildfire season growing longer and more severe in recent years, an increasing number of communities are facing health challenges related to smoke exposure. To better understand how such events might put children at greater risk, EPA researchers and their partners conducted the first study to examine associations between gestational and postnatal exposure to wildfire smoke and prolonged use of prescription respiratory medicines.
To do so, they combined publicly available prescription drug data from six fire-prone western U.S. states with assessments of air pollution exposures during “smoke-days.” They did find an association between increases in wildfire smoke and more prescriptions issued: “…an increase in the repeated, prolonged use of lower respiratory medications in the first 3 yr after exposure among children who were exposed to wildfire smoke during the 3rd trimester and the first 12 weeks after birth.”
Overall, the researchers conclude that there are potential long-term benefits to taking protective measures that reduce wildfire smoke exposures to pregnant women and infants.Air Pollution and Preterm Birth: Comparing Averages to Repeated High Exposure Days
A host of studies analyzing data on average air pollution concentrations over time have found associations between exposures during pregnancy and preterm birth (PTB). Less understood, however, are potential associations between PTB and exposure to periodic spikes of air pollutants such as ozone (O3) and particulate matter (PM), which can have high levels of variability over short periods of time.
To address that knowledge gap, EPA researchers and partners conducted a study comparing the two. To do so, they combined data collected from more than 1.3 million births in North Carolina (part of the North Carolina Birth Defect Monitoring Program) with air quality concentrations from EPA’s Fused Air Quality Surface Using Downscaling model. They assigned exposure levels for both trimester-average and daily threshold exceedances of O3 and PM.
Findings suggest that considering average exposures alone might leave out important information for developing air pollution guidelines that are protective of young children.
“When considering the impact of air pollution on birth outcomes, both the magnitude and frequency of exposures should be considered, particularly for ozone which may exhibit greater temporal viability and diurnal peaks,” the researchers note.
3. Air Pollution and the Congenital Heart Defect Tetralogy of FallotEPA researchers and partners conducted an exploratory study looking for evidence of links between tetralogy of Fallot (TOF)—the most common complex congenital heart defect—and prenatal exposures to air pollution. The study focused on how air pollutant exposure might disrupt the biochemistry of DNA signaling during key periods of development.
To do so, they used DNA from newborn residual blood spots from a large North Carolina study to compare 24 infants with TOF against a control group. They then assigned air pollution exposure levels for particulate matter and ozone during obstetric weeks three through eight, a critical window of susceptibility for congenital heart defects.
What they found was that TOF cases tended to have higher exposures to both particulate matter and ozone. In addition, they observed associations between TOF cases and DNA methylation at regions important for metabolism, inflammation, and immune response pathways.
“This study is among the first to assess relationships among air pollutants, DNA methylation, and TOF case status using residual blood spots. This exploratory work sets the stage for larger studies to investigate these relationships,” the researchers concluded.
Sources
- Jardel, H., Rappazzo, K. M., Luben, T. J., Keeler, C., Staley, B. S., Ward-Caviness, C. K., ... & Dhingra, R. (2024). Gestational and postnatal exposure to wildfire smoke and prolonged use of respiratory medications in early life. Environmental Research: Health, 2(4), 045004.
- Mowla, S. J., Krajewski, A. K., Wilkie, A. A., Rappazzo, K. M., & Luben, T. J. (2025). Air pollution and preterm birth: comparing trimester average and repeated threshold exposure metrics in a North Carolina birth cohort, 2003–2015. Journal of Exposure Science & Environmental Epidemiology, 1-11.
- Luben, T. J., Roell, K., Harrington, C. E., Stingone, J. A., Ward‐Caviness, C. K., Desrosiers, T. A., ... & Olshan, A. F. (2025). Using Residual Newborn Blood Spots to Investigate CpG Methylation in Relation to Air Pollution and Congenital Heart Defects. Birth Defects Research, 117(4), e2473.
May/June
Harnessing New Approach Methods to Protect Children's Environmental Health

The field of toxicology continues to advance the technologies and approaches used to predict potential chemical hazards. Traditional methods use laboratory animals such as small rodents, zebrafish, and fathead minnows to explore the potential health risks that exposures to chemicals and other substances pose. The development of new approach methods (NAMs) that harness powerful computer modeling, robot-aided high-throughput screening mechanisms, curated cell lines, and other high-tech advances are ushering in a new generation of faster, far-less expensive chemical screening and safety tests.
Researchers are beginning to tailor NAMs to explore potential risks to the most vulnerable stages of growth and development—making them more promising for advancing children’s environmental health.
Recent examples illustrate how:
- DevTox
EPA researchers are leading the development of model platforms for conducting cell-based (as opposed to whole-animal based) assays to detect chemical risks to pregnant women, their offspring, and other susceptible populations. One such platform is DevTox, designed to rapidly identify potential hazards and characterize toxic effects during the early, critical stages of cell growth and differentiation in human development.1
Recent research evaluating the technical performance of DevTox across four different developmental assay modes is helping researchers refine it for fast, routine screening of potential developmental toxicants.2
- Assessing Chemicals Lacking Data
Agency researchers used a suite of NAMs-based approaches in a study3 screening previously studied chemicals which had little-to-no existing data for indications of endocrine, developmental, neurological, and immunosuppressive effects, all of which carry serious implications for children’s health. “This case study further enables regulatory scientists from different international purviews to utilize efficient approaches for prospective chemical management, addressing hazard and risk-based data needs, while reducing the need for animal studies,” the authors of the study conclude.
- Testing High-Throughput, High-Content Bioassays
A major driving force behind the development of NAMs is the growing need to quickly and efficiently screen chemicals to address emerging public health and ecological concerns.
Agency researchers and partners used EPA-developed high-throughput, cell-based assays to identify and contrast hazards related to a substance commonly used in car tires as a preservative (6PPD) and its degradant (6PPD-quione), the latter of which has recently been identified as the culprit in ongoing deaths of pre-spawn coho salmon following rain events in the Pacific Northwest.
Including assays based on fathead minnow and zebrafish larvae, rainbow trout gills, RNA sequencing, and mammalian cells yielded results that have implications beyond addressing immediate concerns for coho salmon. “Application of the set of high-throughput and high-content bioassays to test the bioactivity of this emerging pollutant has provided data to inform both ecological and human health assessments,” the authors of the study note.
- Chemical Signatures of Common Household Products
Researchers from EPA led a proof-of-concept study4 applying suspect screening analysis to characterize chemicals from common household products. They extracted samples from 92 products in five categories (cotton clothing, fabric upholstery, shampoo, baby soap, and silicon kitchen tools) and ran them through two-dimensional gas chromatography-high-resolution-time-of-flight/mass spectrometry. Then, they created “ingredient signatures” based on the structural and functional characteristics of the chemicals identified in the sample analysis.
The signatures derived can be used to evaluate new and existing products and could help inform exposure assessments and NAM-based bioactivity screening, ultimately leading to a better understanding of potential health risks associated with household products.
Sources
- Gamble, J. T., Hopperstad, K., & Deisenroth, C. (2022). The DevTox germ layer reporter platform: An assay adaptation of the human pluripotent stem cell test. Toxics, 10(7), 392. Read about the publication and find a download in EPA’s Science Inventory.
- Gamble, J. T., & Deisenroth, C. (2025). Profiling assay performance in the DevTox germ layer reporter platform. Current Research in Toxicology, 100223. The published research is available at: https://doi.org/10.1016/j.crtox.2025.100223.
- Friedman, P., Thomas, R. S., Wambaugh, J. F., Harrill, J. A., Judson, R. S., Shafer, T. J., ... & Sobanski, T. (2025). Integration of New Approach Methods for the Assessment of Data Poor Chemicals. Toxicological Sciences. Read the published paper: https://doi.org/10.1093/toxsci/kfaf019
- Stanfield, Z., Favela K., Yau, A., Menn, C., Edrisi, H., Philips, K. A., … & Wambaugh, J. F. (2025), Developing Chemical Signatures for Categories of Household Consumer Products Using Suspect Screening Analysis. Environmental Science & Technology 59 (2), 1354-1366. Learn more about the study and find a link to the publication on EPA's Science Inventory.
Developmental Toxicity of Disinfection Byproducts

Treating water to make it is safe for drinking requires careful management so that the cleaning agents used, such as chlorine, remove germs, but don’t lead to unhealthy levels of disinfection byproducts (DBPs) as they do their job. DBPs have also been associated with birth defects, and several are regulated by EPA.
To further explore these associations, Agency researchers recently conducted a toxicology study of DBP effects on development. To do so, they exposed pregnant rodents to DBPs and then examined pregnancy loss and eye defects in the offspring. Results illustrated relative potencies between different classes of DBPs that may be valuable for refining risk assessments of exposures to DBPs—important considerations for informing Agency actions to fulfill its mandate to safeguard the nation’s drinking water.
Source
Narotsky, M. G., Fuentes, L. S., Ola, O., Willoughby, T. L., & Lucas, K. (2025). Developmental toxicity of disinfection byproducts in F344 rats: Effects on pregnancy maintenance and eye development. Birth Defects Research, 117(1), e2427. Learn more and see a link to the published paper in EPA’s Science Inventory.
March/April 2025
Exploring PFAS Exposures, Toxicity

Per- and polyfluoroalkyl substances (PFAS) have been found to be ubiquitous in the environment and in samples of tissue from both wildlife and humans. Commonly referred to as “forever chemicals” because they do not break down, some PFAS have also been linked to a host of adverse health effects in children, including development delays, low birth weight, accelerated puberty, and behavioral changes.
Drinking water and food are known to be primary routes of exposure to PFAS chemicals, but few studies exist on how household dust might contribute, a potentially important consideration for protecting children’s health due to their hand-to-mouth behavior and increased vulnerabilities from their relatively small and still-developing bodies.
In a unique research project aimed at shedding light on the potential connection between PFAS exposure and household dust, a team of EPA scientists leveraged data from the National Children’s Study (NCS). They analyzed frozen, archived biological material and house dust samples to search for evidence of links between home levels of PFAS and how much was found in the biological samples (PFAS body burden) of those living there. Findings revealed a positive association between the two.
In another study, a team of EPA researchers conducted laboratory-based exposure experiments to track how exposures to two types of long-chain PFAS (PFO4DA and PFO5DoA) effect maternal and fetal health. The study was designed to increase what is known about the toxicity of these two and similar compounds, which have been detected in biomonitoring studies near manufacturing sites in the U.S. and China.
While further research is needed, results might prove to be an early warning signal for children’s health. Both compounds produced adverse health impacts and acute toxicity from short-term exposures to laboratory animals, “indicating need for additional toxicity data to evaluate potential human health risks,” the research team concluded.
Sources:
Wallis, D. J., Miller, K. E., DeLuca, N. M., Thomas, K., Fuller, C., McCord, J., ... & Minucci, J. M. (2024). Understanding prenatal household exposures to per-and polyfluorylalkyl substances using paired Biological and dust measurements with sociodemographic and housing variables. Environment International, 194, 109157. See a summary and a link in the EPA Science Inventory.
Conley, J. M., Lambright, C. S., Evans, N., Bangma, J., Ford, J., Hill, D., & Gray Jr, L. E. (2024). Long-chain perfluoroalkylether carboxylic acids PFO5DoA and PFO4DA alter glucose, bile acid, and thyroid hormone homeostasis in fetal rats from 5-day maternal oral exposure. Environmental Research, 263, 120210.
Investigating Potential Long-term Impact of Gestational Exposures

When it comes to children’s environmental health, timing can mean everything. Exposures to chemicals that might not harm adults can have dramatic, long-lasting adverse health impacts to children if they occur during particularly vulnerable stages of early life. This is particularly true when it comes to brain and other neurodevelopment.
Results from three recent studies, one by grantees support by an EPA Science to Achieve Results (STAR) grant, and the other two by teams of EPA staff researchers and their partners, shed light on this important aspect of children’s health.
1. Organophosphate Ester Exposure and Executive Function
In the first, a team of EPA grantees used data from the Health Outcomes of Measurements of the Environment (HOME) Study to investigate how gestational exposure to organophosphate esters (OPEs), chemicals used as flame retardants and plasticizers in many consumer products, might impact childhood development and executive function years later. Using statistical analysis of HOME data— measurements of gestational OPE urinary metabolites at different stages of pregnancy and at time of delivery, and executive function assessments performed at age 12—the team found limited evidence of a relationship between OPE exposure at birth and executive function during adolescence.
This novel research provides important clues into the potential for OPEs to impact children’s health, and points to the need for further research. “Given the scarcity of epidemiological studies that have focused on executive function as an endpoint for OPE neurotoxicity, future studies can help investigate this research question further, particularly among large birth cohorts with multiple measurements of OPE metabolite concentrations during pregnancy,” state the researchers in the paper presenting their results.
2. Iodine Deficit and Perchlorate
In another study, EPA researchers and their partners tested how a diet deficient in iodine, which plays a critical role in thyroid function and brain development, might lead to increased vulnerability to exposures to perchlorate, a chemical oxidizer used in such things as rocket fuel, fireworks, airbags, and safety flares.
They found that the combination of both iodine deficiency and perchlorate exposure to pregnant rats was associated with transcriptional alterations to the thyroid glands and brains of their offspring. The offspring were found to have structural changes in their brains, and persistent neurobehavioral impairments as adults. “We report an exacerbation of the effects of developmental exposure to perchlorate on the brain by concomitant dietary iodine deficiency,” the authors of the study note.
3. Comparing Biomarkers of Prenatal Methylmercury Exposure
In a third recent study, EPA researchers conducted an extensive review of the scientific literature to compare the biomarkers and determine which would be most apt for assessing effects of exposure to the developmental neurotoxin methylmercury (MeHg) during pregnancy.
By analyzing five papers containing both maternal hair and cord blood data, a team of EPA scientists found that cord blood was often a more sensitive biomarker than maternal hair. Data on maternal blood suggested that it may be the most sensitive of the three; however, data was limited, and further research would be needed to form a conclusion about maternal blood.
The data derived from the literature review could be used to inform future studies on the relationship between maternal MeHg and developmental neurotoxicity (DNT) in children.
Sources:
Vuong, A. M., Percy, Z., Yang, W., Godbole, A. M., Ospina, M., Calafat, A. M., . . . & Chen, A. (2024). Gestational organophosphate esters (OPEs) and executive function in adolescence: The HOME Study. Environmental Research, 263, 120239.
Gilbert, M. E., Hawks, M. G., Bell, K. S., Oshiro, W., Wood, C., George, B. J., ... & Ford, J. (2024). Iodine Deficiency Exacerbates Thyroidal and Neurological Effects of Developmental Perchlorate Exposure in the Neonatal and Adult Rat. Toxics, 12(12), 842.
Kopylev, L., Dzierlenga, M., Lin, Y. S., Nachman, R., Radke, E., Ru, H., & Segal, D. (2024). Which prenatal biomarker is most appropriate for methylmercury dose-response for neurodevelopmental effects? Journal of Toxicology and Environmental Health, Part B, 1–10.
Particulate Matter and Infant Mortality

A robust body of research concludes that long-term exposure to the tiniest bits of air pollution, microscopic particulate matter with a diameter of 2.5 micrometers or less (PM2.5), can significantly increase the risk of adult mortality. Less, however, is known about how PM2.5 exposures impacts the risk of infant mortality. Reducing infant mortality is a primary focus across EPA’s efforts to improve children’s environmental health.
Recently, a team of researchers from the University of Washington’s School of Public Health and EPA published results of a study analyzing the association between increased long-term PM2.5 exposure and infant mortality. The team conducted a case-control study using publicly-available data to create a birth cohort of all infants born in North Carolina between 2003 and 2015—more than 1.3 million births. Mean PM2.5 exposure decreased substantially as air quality improved over the study period.
Analysis pointed to a possible association between infant mortality and PM2.5 exposure, with stronger effects at the higher PM2.5 concentrations for the earlier years of the study, and lower effects in later years with improved air quality. Researchers point out the need for further investigations given the relatively few studies on PM2.5 and infant mortality. They conclude their findings will be important to inform decisions that aim to protect infants’ environmental health with respect to air pollution.
Source: Jampel, S. M., Kaufman, J., Enquobahrie, D. A., Wilkie, A. A., Gassett, A. J., & Luben, T. J. (2024). Association between fine particulate matter (PM2. 5) and infant mortality in a North Carolina birth cohort (2003–2015). Environmental Epidemiology, 8(6), e350.
See a summary and link to the published research paper in EPA’s Science Inventory.
January/February 2025
Advancing the Science of Soil and Dust Ingestion Studies

Soil and dust ingestion are major routes of exposure for pollution and other environmental toxins, especially for children—who not only exhibit behaviors that increase the likelihood of ingesting soil and dust, but due to their relatively smaller size and still-developing bodies are also more vulnerable to potential adverse health impacts.
To advance the science of soil and dust ingestion studies, a team of EPA scientists and their partners conducted a novel meta-analysis of published studies of soil and dust ingestion in the North American region. Their work provides important insight into the three main types of ingestion studies (tracer, biokinetic, and activity pattern), and illustrates how statistical meta-analysis techniques can be used to estimate mean ingestion and confidence intervals. “Results can be used to better estimate population-based exposure and risk,” the authors conclude.
Source: Cohen, J., Hubbard, H., Özkaynak, H., Thomas, K., Phillips, L., & Tulve, N. (2024). Meta-analysis of soil and dust ingestion studies. Environmental Research, 261, 119649.
See the published study at: Meta-analysis of soil and dust ingestion studies - ScienceDirect
Exploring the Maternal and Development Toxicity of Next-generation Chemicals
Sparked by growing evidence of human and wildlife exposure to a new generation of polyfluoroalkyl substances (PFAS), commonly referred to as “forever chemicals,” a team of EPA scientists conducted one of the first studies to explore potential developmental toxicity of two such chemicals: PFO4DA and PFO5DoA.
The team exposed pregnant laboratory rats to the chemicals and then combined blood and liver RNA and small molecule metabolite analyses with dose-response modeling. Results revealed both genetic and metabolic impacts from the exposures in both the pregnant rats and their developing offspring. The work demonstrates potential developmental toxicity of the chemicals tested, especially to the developing offspring.
Source: Jackson, T. W., Lambright, C. S., Evans, N., Wehmas, L. C., MacMillan, D. K., Bangma, J., ... & Conley, J. M. (2024). Exploring maternal and developmental toxicity of perfluoroalkyl ether acids PFO4DA and PFO5DoA using hepatic transcriptomics and serum metabolomics. Science of The Total Environment, 953, 175978.
See the published results in: Exploring maternal and developmental toxicity of perfluoroalkyl ether acids PFO4DA and PFO5DoA using hepatic transcriptomics and serum metabolomics
Exploring Air Pollution's Impact of Children's Health

Since its establishment in 1970, EPA has targeted cleaner air as a top priority for protecting human health, especially for children who can suffer serious, even life-long impacts if exposed to unhealthy air during certain early lifestages, even those before birth. Two recent EPA studies explore the potential impacts of ozone exposure during fetal development.
Air Pollution and Male Reproductive Health and Development
What role does exposure to air pollution play in rising rates of male reproductive disorders? Because epidemiological studies have associated exposure to ozone with reduced sperm quality, EPA researchers conducted a study to explore if carefully timed exposures to ozone would reduce sperm motility and maturation in laboratory rats.
The team sought to determine if key periods of sperm maturation were particularly vulnerable to adverse impacts from ozone exposure. They also sought to identify components of maturing sperm on the molecular level, namely non-coding RNAs, that could be used to better understand the epidemiological evidence.
While the team did not find evidence of impaired sperm motility from their ozone exposures, they did identify a suite of non-coding RNAs associated with sperm motility, warranting further investigation into the role they may play in sperm maturity, and how they might be used as biomarkers.
Source: Chorley, B. N., Klinefelter, G. R., Nelson, G. M., Strader, L. F., Nguyen, H. H., Schladweiler, M. C., ... & Miller, C. N. (2024). Episodic ozone exposure in Long-Evans rats has limited effects on cauda sperm motility and non-coding RNA populations. Reproductive Toxicology, 108631.
See the published study at: Episodic ozone exposure in Long-Evans rats has limited effects on cauda sperm motility and non-coding RNA populations
Ozone Exposure: Developmental Effects in Male and Female Rats
In assessing chemical risks during pregnancy, EPA researchers learned that ozone exposure during pregnancy may impact fetal growth. Researchers next investigated lung health and development in offspring from rats that had been exposed during pregnancy.
The team studied exposure at varying sensitive windows of exposure, including early gestation and adolescence, in both male and female offspring. They found that lung development in females was more significantly impacted by gestational exposure, whereas the males were more affected by adolescent exposure, especially those from exposed rat mothers. Importantly, the study extends connections between maternal exposure to air pollutants and effects on fetal growth to also include atypical lung development, as well as a distinction between results based on sex. These connections add to our understanding of how early life environmental exposures may contribute to pulmonary disease later in life.
Source: Dye, J. A., Nguyen, H. H., Stewart, E. J., Schladweiler, M. C. J., & Miller, C. N. (2024). Sex Differences in Impacts of Early Gestational and Peri-Adolescent Ozone Exposure on Lung Development in Rats: Implications for Later Life Disease in Humans. The American Journal of Pathology 194(9), 1636-1663.
Learn more from the published study at: Sex Differences in Impacts of Early Gestational and Peri-Adolescent Ozone Exposure on Lung Development in Rats: Implications for Later Life Disease in Humans
Learn more about EPA Lead Research to Protect Children's Health.
Virtual Embryo: Advancing Developmental Toxicology
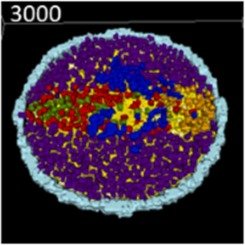
EPA researchers are leading efforts to advance high-tech, computer- and robot-aided technologies and methods for assessing chemical risks. These “New Approaches and Methods” (NAMS) aim to usher in a new generation of toxicology studies that assess chemicals in far faster, more efficient, and less expensive ways than is currently available through tradition, animal-based testing. One area of this work that is particularly promising for children's environmental health is the development of a virtual embryo, which can be used to screen chemicals for their potential to disrupt healthy growth during some of the most vulnerable stages of early life.
A team from the Agency’s Center for Computational Toxicology and partners recently published an important milestone in the development of those efforts: engineering a model simulating key parts of embryonic development. “Here, we engineered a fully computable model of the embryonic disc in the CompuCell3D.org modeling environment to simulate epithelial-mesenchymal transition (EMT) of epiblast cells and self-organization of mesodermal domains (chordamesoderm, paraxial, lateral plate, posterior/extraembryonic),” the authors explain.
The new model advances ongoing efforts to develop a “virtual embryo” model that integrates chemical data with the complex biological systems of development to improve testing. When complete, such a model would greatly expand the capability of researchers to run developmental toxicological experiments.
Source: Barham, K., Spencer, R., Baker, N. C., & Knudsen, T. B. (2024). Engineering a computable epiblast for in silico modeling of developmental toxicity. Reproductive Toxicology, 108625.
See the published study at: Engineering a computable epiblast for in silico modeling of developmental toxicity.
See the current highlights featured on EPA's Chalkboard webpage.

