Statistics for the New Chemicals Program under TSCA
The Toxic Substances Control Act (TSCA) requires EPA to review the potential risks of new chemicals before they enter the U.S. market and, when necessary, put safeguards in place to protect human health and the environment from any risks. Learn more about EPA’s review process for new chemicals.
This webpage provides a general overview of EPA’s new chemicals workload, tracks the status of active cases currently under review by EPA, and illustrates general statistics for all new chemical submissions received since TSCA was amended on June 22, 2016. You can also view statistics related to new chemical submissions prior to the 2016 TSCA reauthorization, New Chemical Program Statistics Prior to June 22, 2016.
EPA takes into account a variety of factors in prioritizing new chemical submissions for review including:
- The date of receipt of submission (e.g., EPA generally strives for a first-in-first-out approach, absent extenuating circumstances)
- Statutory and regulatory deadlines
- The extent to which the submitter has provided additional information DURING the review period – subsequent to the original submission - and the level of effort needed to potentially rework some or all of the risk assessment as a result
- If a submission qualifies for prioritized review related to Executive Order 14318, "Accelerating Federal Permitting of Data Center Infrastructure."
In the last few years, EPA has received an average of about 500 TSCA section 5 notices and other applications each year. This includes premanufacture notices (PMNs), significant new use notices (SNUNs), and microbial commercial activity notices (MCANs) – all of which must be reviewed within 90 days. EPA’s total new chemicals workload also includes applications for exemptions from the full PMN review process (e.g., low volume (LVE), low release and low exposures (LoREX), test market (TME), etc.). These applications represent over 50% of annual applications, have between 30- and 60-day review periods, and are also reviewed by EPA using a robust risk assessment process. EPA review may indicate that an exemption can be granted only if the submitter amends the exemption request to include certain additional limitations on processing, use or disposal. Grants for LVEs only apply to the LVE submitter, any other manufacturer is required to send a new chemical notice before manufacture.
Current Fiscal Year – Cases Completed by Month
| TSCA Section 5 Submissions - Monthly Statistics | |||||||
|---|---|---|---|---|---|---|---|
| FY26 Total (to date) | FY26 Q1 | FY26 Q2 | |||||
| Oct. 2025 | Nov. 2025 | Dec. 2025 | Jan. 2026 | Feb. 2026 | Mar. 2026 | ||
| Newly submitted1 | |||||||
| Notices2 | 51 | 19 | 10 | 17 | 5 | ||
| Applications for Exemptions from Full PMN Review Process3 | 90 | 43 | 15 | 10 | 22 | ||
| Total | 141 | 62 | 25 | 27 | 27 | ||
| Risk Assessments Completed | |||||||
| Notices2 | 40 | 10 | 12 | 11 | 7 | ||
| Applications for Exemptions from Full PMN Review Process3 | 67 | 22 | 19 | 12 | 14 | ||
| Rework Assessments Completed4 | 15 | 11 | 3 | 1 | 0 | ||
| Total | 122 | 43 | 34 | 24 | 21 | ||
| Risk Management Completed5 | |||||||
| Notices2 | 52 | 14 | 8 | 6 | 24 | ||
| Applications for Exemptions from Full PMN Review Process3 | 73 | 17 | 28 | 18 | 10 | ||
| Total | 125 | 31 | 36 | 24 | 34 | ||
| Consent Orders signed by EPA Awaiting Submitter Signature | 10 | 9 | 9 | 9 | |||
| Total Cases Under EPA Review by Month | 9/1/25 | 10/1/25 | 11/1/25 | 12/1/25 | 1/1/26 | 2/1/26 |
| Notices2 | 438 | 442 | 447 | 449 | 460 | 441 |
| Applications for Exemptions from Full PMN Review Process3 | 126 | 121 | 145 | 133 | 123 | 135 |
| Total Cases Under EPA Review | 564 | 563 | 592 | 582 | 583 | 576 |
1Monthly newly submitted case counts include cases that are deemed valid by the 1st of the following month. Additional submissions may subsequently be deemed valid, invalid, or incomplete during EPA’s prescreening and new chemical review process. Final totals of valid submissions received by fiscal year are provided in the “Valid Submissions Received by Fiscal Year” table on this webpage.
2Premanufacture (PMNs), significant new use (SNUNs), and microbial activity notices (MCANs).
3Low volume (LVEs), low release and exposures (LoREXs) (and modifications), and test market (TMEs); and TSCA environmental release application (TERA), and Tier 2 biotech exemptions.
4In June 2024, EPA started reporting the number of rework assessments completed monthly beginning with January 2024 . Rework includes work that supplements completed initial risk assessments, e.g., evaluation of new information from the submitter and/or development of new assessment reports or memos in response to new information or questions.
5Includes consent orders, not-likely determinations, and withdrawals.
PMNs, SNUNs, and MCANs under Review by EPA (as of 2/1/2026 cases total)
- Total Active Cases: 441
- Total Active Cases with EPA: 306
- Total Active Cases with Submitters: 135
There are 441 PMN/SNUN/MCAN cases in the chemical review process; of these, approximately 244 cases are in the risk assessment phase, 62 cases are in the risk management phase, 75 cases are awaiting additional information from the submitter, and 60 cases are awaiting the submitter signature for an Order.
The graphic below describes the number of active cases (PMNs, SNUNs, MCANs) currently under review by EPA and their stage of review.
LVEs, LoREXs, and TMEs under Review by EPA (as of 2/1/2026 cases total)
- Total Active Cases: 135
- Total Active Cases with EPA: 110
- Total Active Cases with Submitters: 25
Under TSCA section 5(h)(4), EPA may, upon application and by rule, exempt a manufacturer of a new chemical substance from the standard PMN review process described under section 5 of TSCA if the agency determines that the chemical will not present an unreasonable risk. EPA has established such regulations for a variety of circumstances. For example, under the LVE regulations at 40 Code of Federal Regulations (CFR) 723, EPA may grant an entity’s application to manufacture new chemicals produced at low volumes (production volume 10,000 kg/year or less) if EPA determines the new chemical will not present unreasonable risk. More information on exemption applications is available here.
There are 135 LVE/LoREX/TME cases in the chemical review process; of these, approximately 108 cases are in the risk assessment phase, 2 cases are in the risk management phase, and 25 cases are awaiting additional information from the submitter.
The graphic below describes the number of active cases (LVE/LoREX/TME) currently under review by EPA and their stage of review.
To see information about a specific case, look up the case number in the exemptions table here.
PMN/MCAN/SNUN Reviews by Fiscal Year
TSCA requires EPA to review submitters' Section 5 notices and make an affirmative finding on the safety of new chemical substances or significant new uses of chemicals (identified by EPA in rulemaking; Significant New Use Rule (SNUR))) submitted under section 5(a) of TSCA before they can proceed to the marketplace. The law sets forth five possible determinations under Section 5 with related actions and these determinations can be broken up into three categories, including:
Allowed to commercialize without restrictions:
- Not likely to present an unreasonable risk – TSCA Section 5(g) notice and, if applicable, accompanying SNUR
Allowed to commercialize with restrictions pending information development, if applicable:
Possible determinations:
- Insufficient information – TSCA Section 5(e) order and accompanying SNUR;
- May present an unreasonable risk – TSCA Section 5(e) order and accompanying SNUR;
- Substantial production/exposure – TSCA Section 5(e) order; and
- Presents an unreasonable risk -TSCA Section 5(f) order and accompanying SNUR.
Not allowed to commercialize pending development of information; and prohibited from commercialization:
Possible determinations:
- May present an unreasonable risk – TSCA Section 5(e) order, testing required before commercialization;
- Insufficient information – TSCA Section 5(e) order, testing required before commercialization; and
- Presents an unreasonable risk – TSCA Section 5(f) order or Section 6(a) rule.
The types of determinations made by EPA per fiscal year for Section 5 notices are provided in the table below.
| Total | 20161 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 20262 | |
| Allowed to commercialize without restrictions | 794 | 23 | 80 | 42 | 267 | 177 | 79 | 34 | 20 | 40 | 27 | 5 |
| Allowed to commercialize with restrictions pending information development, if applicable | 1087 | 2 | 271 | 151 | 79 | 92 | 53 | 83 | 86 | 132 | 101 | 37 |
| Not allowed to commercialize pending development of information; and prohibited from commercialization | 23 | 0 | 6 | 0 | 2 | 1 | 4 | 0 | 0 | 103 | 0 | 0 |
| Withdrawn | 491 | 10 | 128 | 69 | 54 | 49 | 48 | 30 | 39 | 34 | 20 | 10 |
| Total Completed | 2395 | 35 | 485 | 262 | 402 | 319 | 184 | 147 | 145 | 216 | 148 | 52 |
| Invalid or Incomplete | 161 | 8 | 31 | 30 | 24 | 19 | 17 | 13 | 6 | 8 | 4 | 1 |
1Fiscal Year 2016 includes the cases in house at and after the time the Lautenberg Amendments to TSCA were passed but does not include the cases completed prior to the time the Lautenberg Amendments to TSCA were passed (June 22, 2016).
2As of February 1, 2026.
3On March 21, 2024, the U.S. Court of Appeals for the Fifth Circuit vacated nine of these ten orders. A link to the vacated orders can be found here.
Exemption Reviews by Fiscal Year
| Total | 20161 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 20262 | |
| Granted | 2165 | 64 | 415 | 249 | 288 | 227 | 158 | 155 | 157 | 164 | 220 | 68 |
| Denied | 374 | 17 | 107 | 5 | 0 | 2 | 36 | 119 | 47 | 15 | 22 | 4 |
| Withdrawn | 178 | 3 | 33 | 13 | 21 | 12 | 47 | 29 | 2 | 11 | 6 | 1 |
| Total Completed | 2717 | 84 | 555 | 267 | 309 | 241 | 241 | 303 | 206 | 190 | 248 | 73 |
| Invalid or Incomplete | 184 | 13 | 26 | 39 | 19 | 21 | 11 | 17 | 12 | 4 | 18 | 4 |
1Fiscal Year 2016 includes the cases in house at and after the time the Lautenberg Amendments to TSCA were passed but does not include the cases completed prior to the time the Lautenberg Amendments to TSCA were passed (June 22, 2016).
2As of February 1, 2026.
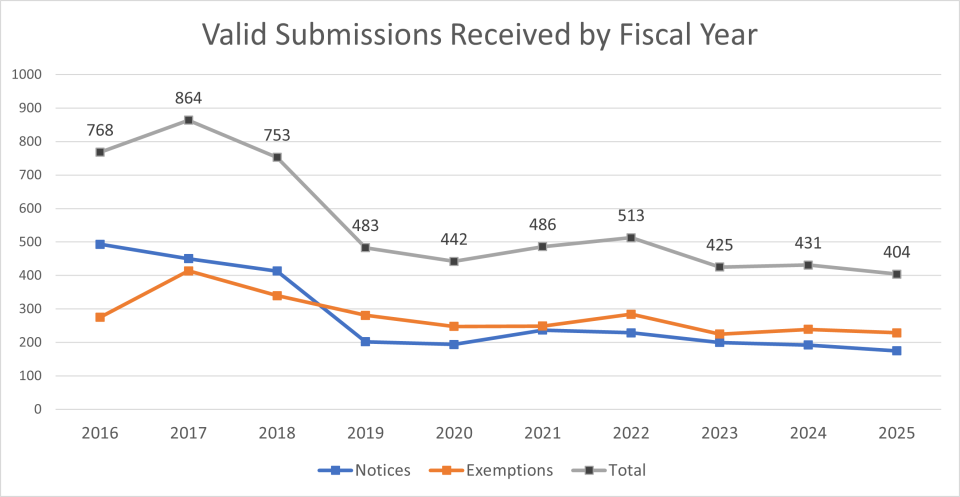
Valid Submissions Received by Fiscal Year
| Submission Type | Total | 20161 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | 2024 | 2025 | 20262 |
| PMN | 2515 | 454 | 416 | 371 | 178 | 174 | 196 | 192 | 169 | 167 | 152 | 46 |
| MCAN | 213 | 29 | 21 | 31 | 19 | 13 | 32 | 28 | 8 | 18 | 13 | 1 |
| SNUN | 108 | 10 | 13 | 11 | 5 | 7 | 9 | 9 | 23 | 7 | 10 | 4 |
| Notices Total | 2836 | 493 | 450 | 413 | 202 | 194 | 237 | 229 | 200 | 192 | 175 | 51 |
| LVE3 | 2771 | 269 | 395 | 324 | 279 | 239 | 237 | 278 | 215 | 231 | 221 | 85 |
| LoREX3 | 28 | 2 | 1 | 6 | 1 | 6 | 1 | 1 | 6 | 3 | 0 | 1 |
| TME | 19 | 1 | 12 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| TERA | 14 | 0 | 2 | 1 | 1 | 1 | 3 | 2 | 2 | 1 | 1 | 4 |
| Tier I | 24 | 0 | 2 | 5 | 0 | 2 | 6 | 0 | 2 | 3 | 4 | 0 |
| Tier II | 13 | 3 | 2 | 1 | 0 | 0 | 2 | 2 | 0 | 0 | 3 | 0 |
| Exemptions Total | 2873 | 275 | 414 | 340 | 281 | 248 | 249 | 284 | 225 | 239 | 229 | 90 |
| Notices of Commencement4 | 1494 | 50 | 289 | 224 | 167 | 190 | 114 | 117 | 85 | 124 | 106 | 29 |
1Fiscal Year 2016 includes the cases in house at and after the time the Lautenberg Amendments to TSCA were passed but does not include the cases completed prior to the time the Lautenberg Amendments to TSCA were passed (June 22, 2016).
2As of February 1, 2026.
3Includes Modifications of prior LVE & LoREX exemption notices.
4The number of Notices of Commencement to manufacture or import (NOCs) received during the listed Fiscal Year.
Note: the PMNs for which notices were received in one Fiscal Year were not necessarily reviewed by EPA in that Fiscal Year.