Processing Fish Fillet Samples
This page outlines the steps for preparing fish fillet homogenate samples for analysis. Wear powderless, disposable gloves when handling fish for tissue processing, changing gloves between each sample.
1. Inspect individual fish.
- Unwrap and inspect fish to ensure that they have been properly preserved during shipment and storage.
- Discard any specimen deemed unsuitable for processing (e.g., thawed, severely damaged, mutilated fish, or fish with skin lacerations, wounds or anomalies that expose muscle tissue or viscera) and note on the sample processing record.
2. Weigh individual fish.

- Use a balance to determine the wet weight of each individual fish sample to the nearest gram. Record weights on the sample processing record. Check balance calibration at the beginning and end of each weighing session and after every 20 weighings in a weighing session.
- Weigh fish on a foil-lined balance tray. Replace the foil lining after each weighing to prevent cross contamination between samples.
- As the fish thaws, keep all liquid as part of the sample.
3. Remove otoliths, scales, spines or maxillary bone for age determination (optional).
- Age provides a good indication of the duration of exposure to pollutants.
- Age may be determined by a fisheries biologist using otoliths, scales, spines or in some cases, maxillary bones.
- Scaleless fish (catfish, etc.) - clip pectoral fin spines.
- For Lake Trout, studies found that thin sections of maxillary bone (upper jaw) are easier to read and just as accurate as otoliths (Retherford, 2014 and Wellenkamp and He, 2015).
- Store the otoliths, scales, spines, or maxillary bones in small envelopes or plastic bags labeled with the tissue specimen identification number. Note the removal of otoliths, scales, spines, or maxillary bones from each fish on the sample processing record.
4. Determine the sex of each fish (optional).
- Fish sex should be determined by a knowledgeable fisheries biologist.
- Make an incision on the ventral surface of the body from a point immediately in front of the anus toward the head to a point immediately posterior to the pelvic fins. If necessary, make a second incision on the left side of the fish from the initial point of the first incision toward the dorsal fin. Incisions should not interfere with cutting out the fillet.
- Fold the resulting flap back to observe the gonads. Ovaries range from granular in texture (in immature stages) to fully developed eggs, and their color can vary widely depending on the species (e.g., white, golden brown, pink, orange, or red). Testes appear uniform in texture and typically white or cream in color. (Fish Necropsy Manual)
- Record the sex of each fish on the sample processing record.
5. Note morphological abnormalities (e.g., deformities, eroded fins, lesions, and tumors).
- Assessment of gross morphological abnormalities in finfish can be conducted in the field or during initial inspection at the processing laboratory prior to filleting and should be noted on the field record form.
- When documenting morphological abnormalities, consult resources such as Sinderman (1983) or Smith et al. (2002).
6. Remove scales from fish with scales and remove skin from scaleless fish (e.g., catfish).
- Use separate sets of utensils and cutting boards for scaling/skinning fish and for filleting fish to avoid sample contamination.
- Lay fish flat on a clean stainless steel or glass cutting board.
- Fish with scales - remove the scales and adhering slime by scraping from the tail to the head using a stainless-steel fish scaler or the blade edge of a stainless steel, ceramic, or titanium knife.
- Fish without scales - remove the skin by loosening the skin just behind the gills and pulling it off between knife blade and gloved thumb. This method of removing skin may vary by species.
- Wash the outside of the fish with distilled water (or another form of contaminant-free water) and place on a second decontaminated cutting board for filleting.
- Control cross-contamination between composites by following the decontamination procedures described in the Equipment subsection before processing each new composite of fish. Rinse the cutting board and knife with distilled water (or another form of contaminant-free water) before processing each fish within a composite.
7. Fillet (resection).
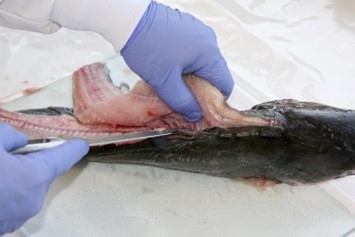
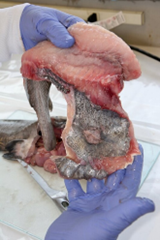
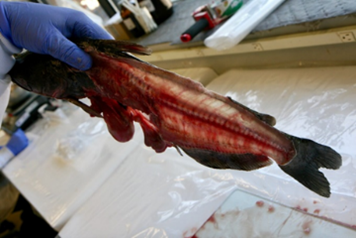
- An experienced fisheries biologist or trained laboratory staff member should fillet the fish or train others in the technique.
- Wash hands with soap and rinse with tap water, followed by distilled water. Wear gloves during the entire filleting process and change gloves before processing either a new individual fish or a new group of fish for a composite. Gloves should be talc- or dust-free, and of noncontaminating materials (e.g., nitrile gloves).
- Care must be taken to avoid contaminating fillet tissues with material released from inadvertent puncture of internal organs. If this happens, the fillet tissue should be eliminated as a sample or rinsed in distilled water (or another form of contaminant-free water). Add a notation to the sample processing record.
- Use clean, high-quality stainless steel, ceramic, or titanium knives to remove fillets from each fish.
- Include the belly flap with each fillet. Any darker muscle tissue in the vicinity of the lateral line just under the skin, should not be separated from the light muscle tissue that constitutes the rest of the muscle tissue mass.
- Carefully remove any bones still present in the tissue after filleting. Use the tip of the fillet knife or a clean pair of forceps.
- Remove both fillets from a fish and combine them for homogenization.
8. Weigh fillets to the nearest gram and record the mass on the sample processing record.
9. Homogenize fillets.



- Grind and homogenize fillets to ensure even distribution of contaminants throughout tissue samples and to facilitate extraction and digestion of samples.
- Use a stainless-steel grinder, high-speed blender, or homogenizer to grind and homogenize fish fillets. Large fillets may be cut into smaller cubes with high-quality stainless steel or titanium knives prior to homogenization. Parts of the blender or homogenizer used to grind the tissue (i.e., blades, probes) should be made of tantalum or titanium rather than stainless-steel.
- For best results, grind and homogenize when the biological tissue is still partially frozen. Additionally, chilling the grinder/homogenizer briefly with a few chips of dry ice will reduce the tendency of the tissue to stick to the grinder. These techniques will make grinding easier (Stober, 1991).
- Grind the fillet sample into a stainless-steel or glass bowl until the tissues appear to be homogeneous. Repeat as necessary until the tissue is a uniform color and finely ground texture. Do not let the homogenate heat up in the process.
- Skin-on fillets of some finfish species are especially difficult to homogenize completely. No chunks of tissue or skin should remain in the sample homogenate because these may not be extracted or digested efficiently and could bias the analytical results. If complete homogenization of skin-on fillets for a particular target species is a chronic problem or if local consumers are likely to prepare and consume skinless fillets of the species, researchers may consider analyzing skinless fillet samples.
- Note the preparation specifics for each homogenate on the sample processing record.
10. Prepare a composite homogenate.
- Combine all fillets from the fish that make up a composite together making sure to thoroughly mix the tissue.
- It is essential that the mass of the composite homogenate yields adequate mass to perform all targeted analyses. Mass requirements vary based on:
- The number of target chemicals that will be analyzed
- The amount of tissue required for analyses
- Sample size requirements may vary among laboratories and the analytical methods used. Consult with the analytical laboratory supervisor to determine the actual weights of homogenates required to analyze for all selected target analytes.
- Label containers with aliquot identification number, sample weight (to the nearest 0.1 g), and date aliquots were prepared.
- Weigh aliquots of the homogenate to meet the analytical laboratory needs for each particular target analyte or lipids analysis and store the analytical aliquots (as described in each method analysis) prior to shipment to the laboratory.
- Freeze the remainder of each composite homogenate (i.e., tissue in excess of that needed for analysis) at ≤-20 °C to archive it. Record the designation "Archive" and the preparation date on the sample label. Record the location of the archived samples on the sample processing record under "Notes." Archived samples provide a valuable backup if samples are lost in transit or there are analytical problems.


