How to Apply
To successfully apply for an equivalency recommendation:
- Notify the Pathogen Equivalency Committee (PEC) of your intent;
- Develop a sample;
- Create an analysis plan to gather data in support of your process's equivalency;
- Carry out that sampling plan; and
- Complete and submit an official pathogen reduction equivalency application package.
The PEC will then evaluate the package and make its recommendation through the appropriate channels. Figure 1 demonstrates the typical equivalency recommendation process.
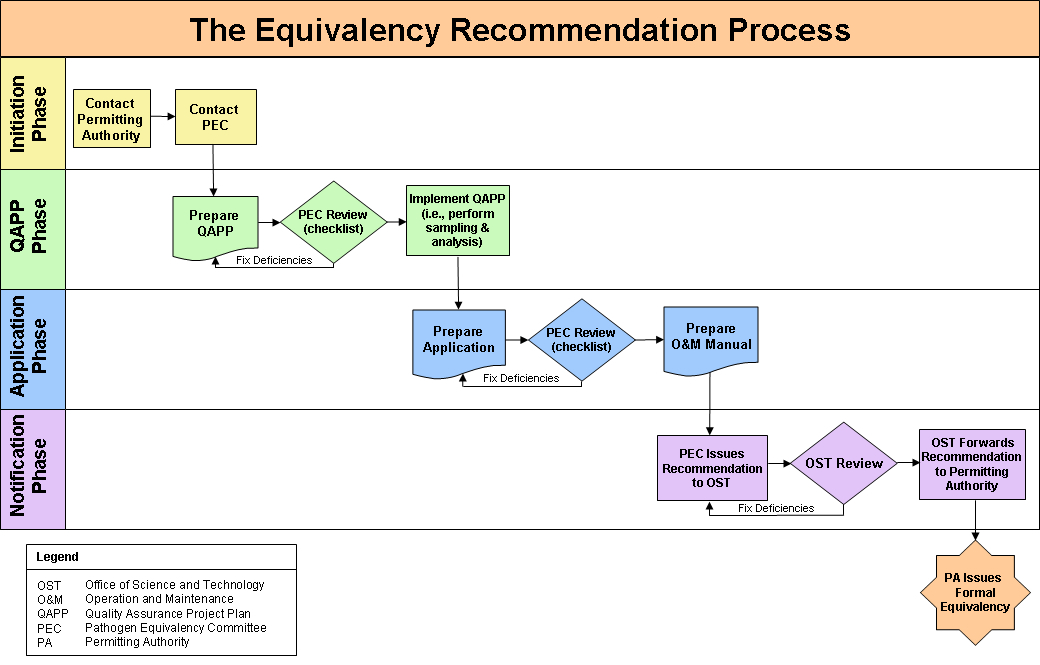
The process can be broken down into twelve steps with four main phases:
Resources
Applicants may use the following resources as guidance when submitting an application package to obtain a recommendation for equivalency to a Process to Further Reduce Pathogens (PFRPs) or a Process to Significantly Reduce Pathogens (PSRP).
- Application Guidelines
- A detailed outline for a full application for an equivalency recommendation. The outline is annotated, providing specific information appropriate for each section.
- Quality Assurance Project Plan (QAPP)
- A QAPP ensures that the desired quality in sample collection, laboratory analysis, data validation & reporting, and documentation & record-keeping are achieved and maintained
- EPA Regional and State Contacts for Biosolids
- Identify your permitting authority and access their contact information.
-
Completeness Checklist (doc)
- This checklist is used by the PEC to review submitted QAPPs and equivalency applications. It is provided to help applicants double-check that all required and applicable elements have been addressed in their QAPP/equivalency application before submittal.
- Equivalency Application Package (doc)
